IFOP develop a study on intraperitoneal use of oxitetracycline in salmons
October 11th, 2016
Luis Norambuena
On September 28, at the Patagonia Hall of Gran Pacifico Hotel in Puerto Montt, it was carried out the diffusion workshop on the results of FIPA project No. 2014-91. It was attended by representatives of the national authority, the productive sector, service laboratories and pharmaceutical companies.
Luis Norambuena researcher of the Department of Hydro biological Health of Aquaculture Research Division of IFOP, said “oxitetracycline is the second antibiotic primarily used in Chile for the treatment of Piscirickettsiosis, which is in turn by far the leading cause of mortality salmon (due to infectious causes) in the country.
Treatment with this antibiotic is carried out orally through food. The main disadvantage of this form of administration is the low absorption of the antibiotic from the fish, as well as the antibacterial amount released to the environment.
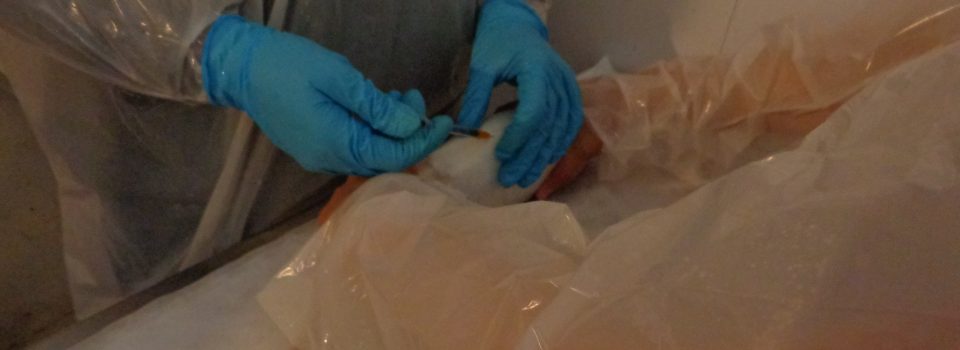
As an alternative to oral treatment, injectable administration intraperitoneally is being used. The advantage of this type of treatment is that the fish take advantage more efficiently, so, a much smaller quantity of antibiotics could be utilized, with the consequent benefits to the environment. With this type of treatment, higher concentrations of oxitetracycline in the body could be achieved for a long time, so the treatments could be more effective.
The main problem with this type of treatment it is that the time after applying the treatment expected could be very long, considering the levels allowed of oxitetracycline concentration in the final product for human consumption and depending on the market product destination.
In general, there is no effect or risk to humans, since the salmon consumed in normal amounts, it does not have trace levels of the product, given the sampling and approval incurred by the national authority previous to export, as well as the surveillance carried out upon arrival to importing countries though residual control programs.
Among the main results and conclusions are:
Oxitetracycline concentration achieved in the muscle would actually be greater than that achieved with oral treatment, which would remain high for a prolonged period of time, being the treatment more effective.
With the use of this type of treatment, a lower amount of oxitetracycline could be used effectively, being less the ingress into the environment.
The waiting time to harvest fish after OTC intraperitoneal treatment would be very long, could be 5 months or more, depending on the target market and water temperature.